Coordinator:
Dra. Márcia Motta Maués • Embrapa Eastern Amazon (e-mail: marcia.maues@embrapa.br)
Protocol Researchers:
Amapá - Dr. Alexandre Luis Jordão (IEPA)
Dr. Carlos Alberto Hector Flechtmann (UNESP - Ilha Solteira/SP) - Collaborator
East Pará - Dr. Hermes Fonseca de Medeiros (UFPA-Altamira)
Maranhão - Dr. Francisco Limeira de Oliveira (UEMA-Caxias)
Mato Grosso - Dr. Evandson José dos Anjos Silva (UNEMAT-Cárceres)
West Pará - MSc. Yukari Okada (UFOPA)
Groups of interest and diversity of species evaluated by the collection site:
Apidae - 120 species;
Drosophilidae - 40 species;
Mesembrinellidae - 10 species;
Nymphalidae - 40 species;
Tephritidae - 15 species;
Scolytinae and Platypodinae (Curculionidae) - 100 species.
Biological role of the group:
Flies are part of the Diptera order, comprising 45 different families, commonly referred to as flies. Flies that utilize decomposing organic vegetable material for feeding and reproduction purposes carry out an important role as saprophagous organisms in the decomposition and nutrient recycling processes. Some of these insects can feed and reproduce on mature fruits, acting as herbivores and playing a very important economic role as pests within the farming industry (TIDO et al., 2003). However, various species may also play a role as pollinators through the utilization of flowers for feeding and as an oviposition substrate (SILVA; MARTINS, 2003). Various degrees of association between flies and man can also be observed, from complete association, to situations in which species only occur in pristine environments. This susceptibility in relation to environments, allied to the speed of response in terms of populations, provides the fly with the particular capacity of functioning as an indicator of human interference within natural environments (MARTINS, 2001).
Bees are insects belonging to the Hymenoptera order and Apidae family, being estimated that more than 30,000 different species exist around the world. Besides being part of one of the richest groups within the Neotropical region in terms of species, bees also contribute to the diversity of the organisms to which they come into contact by means of cross-pollination. Among the roles of this insect we can highlight the pollination of a large number of vegetable species, both native and cultivated (OLIVEIRA; MORATO, 2000; GREENLEAF; KREMEN, 2006), acting as a disperser of fruits and, very probably, a disperser of spores and fungi (Eltz et al., 2002). Furthermore, many species have their own products such as honey and wax, mainly being used as food, for pharmacopoeia purposes and for supplementing family incomes.
Butterflies are represented by five families; Hesperiidae, Papilionidae, Pieridae, Lycaenidae and Nymphalidae. The adult form is active by day, feeding on decaying material, plant exudates and nectar. Butterflies that feed on fermenting fruit make up approximately one third of the butterfly species within a Neotropical forest. These butterflies belong to the Nymphalidae family and are the only group of butterflies that can be collected through traps. Taxonomically speaking, the group is very well known and can be used in surveys, rapid ecological analyses and for monitoring purposes. The butterfly can also be used as a bio-indicator in relation to environmental changes due to its intimate relationship with vegetation during its larva and adult phases (FREITAS, 2008). The fauna inventory of frugivorous butterflies is essential in understanding more about the community, in addition helping us develop mechanisms for the insect's preservation (FERRO; DINIZ, 2007).
Beetles of the sub-families Scolytinae and Platypodinae (Curculionidae) are usually tree borers. Within these groups we find the most evolved species in Curculionidae, where several species cultivate their own food, while some others show maternal care. These borers are a large group, with over 7,000 described species, and found typically in forests. In natural forests, while attacking and developing into stressed or dying hosts, act as important in nutrient recycling. However in reforestation these borers are serious pests, and they are amongst the most important living organisms that inflict damage to these forests. Besides the ecological importance of these groups in natural environments and the economic importance in reforestations, they are important as bioindicators of environmental degradation. Several of these are known as interior species, and they are usually present only in well preserved areas. Conversely, there are also border species, whose presence indicate anthropized or disturbed areas. Hence, these beetles serve as an excellent tool to indicate the degree of preservation of an habitat.
A) TECHNIQUE 1 - FLY TRAPS WITH BANANA BAIT
Banana bait is used in the traps shown in figure 1. The bait is prepared the day before with ripe, mashed bananas until a homogenous substance is obtained. Each trap is loaded with around 100 ml of banana bait, set in a hanging position approximately 30 cm from the ground, remaining in the field for 48 hours. After this period, the animals found within the traps are collected through use of a manual vacuum device.
This method is mainly focused on collecting the Drosophilidae, but can capture two target groups: Drosophilidae and Mesembrinellidae.

Figura 1. Banana bait trap.
TECHNIQUE 2 - FLY TRAPS USING MOLASSES BAIT
Molasses bait is used in the traps shown in figure 2. This bait is an aqueous solution containing 10% molasses and 1% borax. The bait must be prepared on the same day as the traps are placed in the field. Each trap is loaded with around 150 ml of molasses bait, set in a hanging position approximately 150 cm from the ground, then remaining in the field for 48 hours. After this period, the bait is poured out into a fine sieve so that the animals that have fallen into the liquid may be collected.
This method is mainly used for the Tephritidae, but can also be used for the groups mentioned in technique 1.
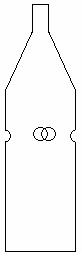
Figure 2. Trap for molasses syrup bait. This simple process consists of a plastic 2 liter bottle, in which four holes are made in the side, each 2 cm in diameter. The molasses syrup solution is then deposited at the bottom of the bottle.
C) TECHNIQUE 3 - FLY TRAPS WITH HYDROLYZED PROTEIN BAIT
Handmade traps will be used, each trap consisting of a 1.5 liter plastic bottle with four 2 cm diametrically opposed holes in the side, containing 300 ml of hydrolyzed protein and stabilized with 5% borax, the traps then being distributed across the 30 plots with even spacing of 40 meters between each bottle, each plot having a total of three traps hung 1.80 meters above the ground, totaling 90 (ninety) traps across the site.
The collected samples are placed in flasks containing 70% alcohol, labeled with the number of the respective trap, the area in which the trap was positioned and the date of the inspection.

Figure 3. Lung trap with bait. This trap consists of a small 500 gram tin covered with transparent plastic material. These tins have side perforations where the flies enter, an inverted paper funnel being placed over the tin, access then being gained to the upper area, covered by the plastic material, through this funnel.
D) TECHNIQUE 4 - TRAPS WITH BAIT FOR ORCHID BEES (Euglossine bees)
The bait consists of essences, placed within the trap's cotton material at the time of placing the trap within the field (NEMÉSIO; SILVEIRA, 2006). The amount of essence placed within the cotton depends on the type of essence used, each essence having its own varying level of bee attracting power (CARVALHO et al., 2006; ZIMMERMANN et al., 2006). The type of bait is methyl salicylate. Other attractive essences may also be used, such as eucalyptol, cineole, vanillin and methyl cinnamate.
It is necessary for the bait to maintain its constant attractiveness during a period of more than 24 hours so that a full sampling array or design can be achieved and so that the spatial effect is not confused with the circadian variation effect in the animal’s activity. This effect was obtained during a protocol evaluation collection through the use of 2ml of methyl salicylate. Other attractive essences may also be used as alternatives to the sampling pattern described below in order to supplement collected samples, essentially aimed at increasing the list of species obtained.
Each trap is positioned approximately 1.5 m above the ground, remaining in the field for a period of 24 hours. After this period the trap is then closed and the bee specimens removed. Removal of the bees is simply a case of closing the entry points to the trap and waiting for the captured animals to die, intoxicated by the bait itself, or alternatively, by inserting a new substance to kill the animals. In the event of a latter scenario, it is necessary to ensure that the substance used leaves no residue, prejudicing subsequent use of the traps.
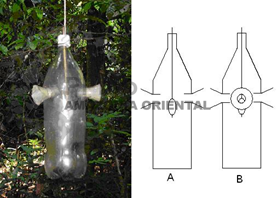
Figure 4. Trap for Euglossine Bees. A funnel made from a plastic bottle neck is inserted into each one of the holes. The funnel is internally coated with sand, affixed with Styrofoam glue and placed in the holes. A wooden barbecue skewer is inserted into the bottle’s lid securing the wad of cotton wool that contains the bait.
Sampling unit: The result obtained using a trap, over a collection period of 24 hours.
Sampling design: Each of the plots will have the same number of traps. Four traps of each type will be prepared for techniques 1, 2 and 3, for each plot (Figure 5). Technique 1 is used in conjunction with collection technique 2, maintaining an even level of distribution and interposing the two methods. The other two techniques are placed together, the Euglossine bee traps being placed 200 meters apart.
Additional important environmental data for this group: Microclimatic data such as temperature, light, rainfall and humidity are very important in terms of this protocol's target group. Micro meteorological stations can be used for obtaining this type of data. Each plot of land will be monitored for 48 hours, coinciding with the exposure time of the traps; a total of three micro weather stations being randomly distributed throughout the plot.
The method of preserving the collected material: The specimens found within the banana and molasses syrup traps will be preserved in 70% alcohol, done so until 10 pairs of each species are found. In the case of drosophila (fruit flies), some of the specimens will be kept alive in isofemale stocks in order to facilitate taxonomic identification and for future study requirements (DAVID et. al., 2005). Any orchid bees that are not already dead when extracted from the traps will be sacrificed and then stored in blister-type envelopes with the necessary explanatory notes, afterwards housed in aluminium containers with a few drops of ethyl acetate in order to prevent fungal attacks or rot. Specimens will be mounted using entomological pins, as well as being labeled and identified. All non-identified unicatas and specimens will be stored at the respective specialist institutions, with the remaining species being part of the available reference collections for all participating PPBio organizations, providing that minimum conditions are available for specimen conservation. All specimens, preserved in alcohol or pinned and mounted, will be housed in the collections of the participating organizations.
E) TECHNIQUE 5 - TRAP WITH FRUIT BAIT FOR COLLECTION OF BUTTERFLIES
Each trap consists of a cylinder with closed nylon screening at the top, including a metal frame at the top and bottom areas. The cloth screen has a vertical opening of 50 cm running along its length, closed with a Velcro strip and used for taking the butterflies out of the trap. Suspended from a ring in the lower area of the cylinder is a square piece of wood hanging about 5 cm below the cylinder’s opening. A small plastic container with mashed banana paste, fermented for more than 48 hours, is placed on the square section of wood at the bottom of the cylinder, used as the bait for attracting the butterflies. A more detailed description of this trap can be obtained in DeVries (1988).
The banana bait is prepared using ripe bananas which are then mashed into a homogenous pulp, done so at least three days before collection takes place. The fermentation process is natural, with no need to add biological yeast. Each trap is filled with approximately 100 ml of bait.
The traps are hung by string from low branches in the forest’s understory, around 1.0 to 1.5 m above the floor. No vegetation is cut or changed in any way while placing the traps. The collection period in each plot will last for 5 consecutive days. The traps will be visited on a daily basis, with fresh bait being added on the third day. Butterflies are to be removed with pincers every 24 hours.
Sampling unit: The result obtained through use of a trap over a period of 24 hours.
Sampling design: Six traps will be evenly set out along the main trail of each plot at intervals of 50 m (0 m, 50 m, etc.). The traps will be placed at no more than 5 m from the trail, the collection across all 30 plots taking a total of 15 days, with the sampling team being able to service 10 plots every 5 days. The traps will be set in place for each collection sequence and then packed away between outings. Four collections are planned per year, two during the dry season and two during the wet.
Handling of specimens: Live specimens retrieved from the banana traps will be sacrificed in special flasks containing ethyl acetate and then stored in entomological envelopes with relevant notes, later packed away in plastic recipients containing naphthalene in order to prevent fungal attacks and rot. Specimens will be mounted using entomological pins, then labeled and identified. All non-identified unicatas and specimens will be stored at the respective specialist institutions, with the remaining species being part of the available reference collections for all participating PPBio organizations, providing that minimum conditions are available for specimen conservation.
F) TECHNIQUE 6 - TRAPS FOR SCOLYTINAE/PLATYPODINAE (CURCULIONIDAE)
We use flight intercept traps (Figure 5; BERTI Filho & FLECHTMANN, 1986) to trap these beetles. This trap can also be easily built out of 2-liter clear PET (polyethylene terephthalate) soft drink bottles (Figure 6).

Figure 5 - ESALQ-84 flight impact trap.

Figure 6 - Modified ESALQ-84 flight impact trap, built from a 2-liter clear PET bottle.

Figure 7 - Dispenser with 95% ethanol as the lure.
These traps are baited with commercial ethanol, the most frequently used semiochemical in Brazil (FLECHTMANN et al., 2001). Ethanol is added to vials with ca. 30 ml capacity; a wick is passed through the cap (Figure 7), allowing for volatilization of the ethanol to the environment at a constant release rate via capilarity.
Each trap is set at 1.50 m above the ground, with the reference point in the trap the ethanol vial. The collecting jar is filled with water, to which some detergent is added to break the surface tension of the water; salt might be added to aid in insect preservation when needed. The trap should be checked every seven days, after which trapped insects are removed, the liquid of the collecting jar is replaced and the ethanol is replenished when needed. The collecting funnel inner part should be kept clean from debris and dust at all times.
Sampling design: when you have a homogeneous area, 5 traps are sufficient, evenly distributed along the site. If you site is divided into quadrants, these should be grouped based on similar vegetation and altitude, and 5 traps should be placed within each grouping. Traps should always be placed at least 20 m inside the plot (and away from the border) to avoid edge effects. Trapping frequency is weekly. The ideal is to collect for one whole year, so seasonal variation can be evaluated. However, when this is not the objective of it is not feasible, at least 15 weeks of trapping should be done, and during the rainy season, when these beetles are more abundant.
Handling of the material: trapped insects should be transferred to vials containing 70% alcohol until determination. For each different site a series of at least 10 specimens should be pinned for each morphotype, which should be labeled, with collecting data in English.
Environmental data important for the group: for Scolytinae/Platypodinae, it is important to record daily data for air average air temperature, average air moisture and daily cumulated rainfall. Data should be obtained from the nearest weather.
Restrictions on activities that could prejudice protocol development: Considering that most Diptera and Hymenoptera target groups are attracted to decomposing material, no decomposing organic residue should be allowed in the sampled areas, including leftovers from camping sites. When targeted organisms are Scolytinae/Platypodinae, avoid any areas where there was recent logging activity or debris such as fallen trees originated from storms.
Bibliography and References:
BERTI FILHO, E.; FLECHTMANN, C.A.H. A model of ethanol trap to collect Scolytidae and Platypodidae (Insecta, Coleoptera). IPEF, n. 34, p. 53-56, 1986.
CARVALHO, C.C.; REGO, M.M.C.; MENDES, F.M. Dinâmica de populações de Euglossina (Hymenoptera, Apidae) em mata ciliar, Urbano Santos, Maranhão, Brasil. Iheringia, Sér. Zool., vol. 96, n. 2, p.249-256. 2006.
DAVID, P. G.; LEGOUT, H.; PE´TAVY, G.; CAPY, P.; MORETEAU, B. Isofemale lines in Drosophila: an empirical approach to quantitative trait analysis in natural populations. Heredity, v. 94, p. 312, 2005/2006.
ELTZ, T.; BRÜHL, C.A.; GÖRKE, C. Collection of mold (Rhizopus sp.) spores in lieu of pollen by the stingless bee Trigona collina.Insectes Soc., v. 49, p. 28-30, 2002.
FLECHTMANN, C.A.H.; OTTATI, A.L.T., BERISFORD, C.W. Ambrosia and bark beetles (Scolytidae: Coleoptera) in pine and eucalypt stands in southern Brazil. For. Ecol. Manag., v. 142, n. 1/3, p. 183-191, 2001.
GREENLEAF, S.S.; KREMEN, C. Wild bee species increase tomato production and respond differently to surrounding land use in Northern Califórnia. Biological Conservation, v.133, n. 1, p. 81-87, 2006.
MARTINS, M.B. Guilds of Drosophilids on Forest Fragments. In: BIERREGAARD, R.O.; GASCON, C.; LOVEJOY, T.E. (Orgs.). Lessons from Amazonia. The ecology and conservation of a fragmented forest. Yale: yale University, 2001.
NEMÉSIO, A.; SILVEIRA, F.A. Edge Effects on the Orchid-Bee Fauna (Hymenoptera: Apidae) at a Large Remnant of Atlantic Rain Forest in Southeastern Brazil. Neotropical Entomology, v. 35, n. 3, p. 313-323, 2006.
OLIVEIRA, M.L.; MORATO, E.F. Stingless bees (Hymenoptera, Meliponini) feeding on stinkhorn spores (Fungi, Phallales): robbery or dispersal? Revta. Bras. Zool., v.17, p. 881-884, 2000.
SILVA, A.R.; MARTINS, M.B. Insetos polinizadores de Theobroma speciosum (Sterculiaceae) e conservação da biodiversidade. In:Estação Científica Ferreira Penna - dez anos de pesquisa na Amazônia; contribuições e novos desafios. Belém: MPEG, 2003. (Cadernos e Debates, v. 7).
TIDON, R.; LEITE, D.F.; LEÃO, B.F.D. Impact of the colonisation of Zaprionus (Dipetra, Drosophilidae) in different ecosystems of Neotropical Region: 2 years after the invasion. Biological Conservation, v. 112, p.299-305, 2003.
WOOD, S.L. Bark and ambrosia beetles of South America (Coleoptera: Scolytidae). Brigham Young University, Provo. 900 pp., 2007.
ZIMMERMANN, Y.; ROUBIK, W.D.; ELTZ, T. Species-specific attraction to pheromonal analogues in orchid bees. Behav Ecol. Sociobiol, v. 60, p. 833-843, 2006.